— Delegates urge AMA to rescind policy discouraging the drug for COVID-19 patients; other side falls victim to #INCOMCORRUPT
Despite the evidence that has shown that hydroxychloroquine and chloroquine aren’t effective for patients with COVID-19, a frustrated group of physicians are urging the American Medical Association (AMA) to rescind its March statement that discouraged physicians from prescribing the unproven drug for that purpose, even as an early-stage treatment.
This resolution, led by Atlanta rheumatologist John Goldman, MD, an alternate delegate representing the Medical Association of Georgia, was considered at the AMA Special Meeting of its House of Delegates. He said the March statement, issued jointly by the AMA and two pharmacists’ organizations, was hurting physicians’ ability to help patients infected with the virus.
The link to Resolution 509 for hydroxychloroquine is the following. It starts on page 16. Highlights in red/ comments in purple:
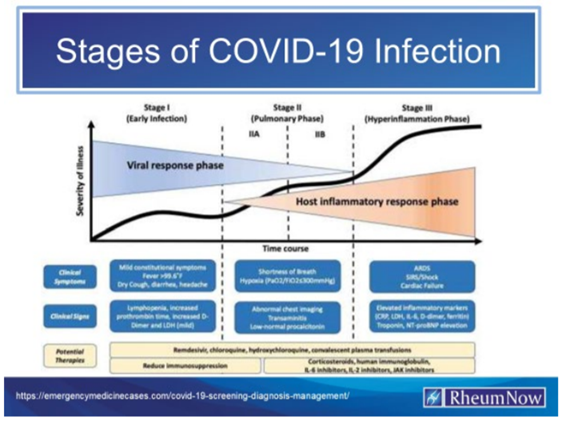
Whereas, During the early infection phase (Stage 1), the virus multiplies inside the body and is likely to cause mild symptoms that may be confused with a common cold or flu; and Whereas, The second phase is the pulmonary phase (Stage 2), when the Immune System becomes strongly affected by infection and leads to primarily respiratory symptoms such as persistent cough, shortness of breath and low oxygen levels. Problems with blood clotting–especially with the formation of blood clots–may be predominant in Stage 2; and Whereas, The third hyperinflammatory phase (Stage 3), occurs when a hyperactivated immune system may cause injury to the heart, kidneys, and other organs. A “cytokine storm”–where the body attacks its own tissues–may occur in this phase; and
Whereas, There is no current Federal Drug Administration (FDA) indication for the treatment of Early Coronavirus infection (and why is that exactly and why are agency heads still employed?), but early emergency use authorization (EUA) originally approved the use of hydroxychloroquine and then rescinded it (ditto) (2); and Whereas, The FDA limited use of convalescence plasma but now has rescinded that limitation (3); and
Whereas, Hydroxychloroquine and Chloroquine are FDA approved medications for over 50 years, and these medications are safely prescribed long-term for other indications (exactly, seems odd doesn’t it?) (2); and
Whereas, AMA President, Patrice A. Harris, MD, issued the following statement: “The AMA is calling for a stop to any inappropriate (nice word choice-message received loud and clear) prescribing and ordering of medications, including chloroquine or hydroxychloroquine, and appealing to physicians and all health care professionals to follow the highest standards of professionalism and ethics” (4); and
Whereas, The AMA, American Pharmacists Association, and American Society of Health System Pharmacists issued a joint statement on March 25, 2020 on inappropriate ordering, prescribing, or dispensing (God forbid we get safe and efficacious medicine that actually works out to Americans) of medications to treat COVID-19 (4); and Whereas, Some states, pharmacy boards and institutions have forbidden the use of these medications for COVID-19 infection (4, 5); and
Whereas, A proposed regimen to treat COVID-19 for Stage 1, includes 10 days of hydroxychloroquine, Azithromycin, zinc, and on occasion Vitamin D (6); and
Whereas, This regimen is not being advocated for Stage 2 and Stage 3 COVID therapy (who was concerned about competition with remdesivir and why and were they compensated?–paging Antitrust Division) ; and Whereas, The original studies published in The Lancet and The New England Journal of Medicine (NEJM) initially citing harm due to hydroxychloroquine and chloroquine use were
retracted by said journals (now said journals sans reputations) due to dubious research methodology and incorrect conclusions (7, 8, 9); and
Whereas, AMA policy H-120.988, “Patient Access to Treatments Prescribed by Their Physicians,” supports a physician’s autonomy to prescribe medications the physician believes to be in the patient’s best interest, where the benefits outweigh risk and the patient consents; and Whereas, Physicians have used off label medications for years and this use is supported by existing policy; and
Whereas, Data regarding harm have been limited due to poorly designed studies or studies usually in Stage 2 or later, or stopped without harm but no effect in phase 2 and hypothesis (sabotaged studies yes, possibly research fraud) (7, 8, 9, 10, 11, 12); and
Whereas, There are many studies that indicate that the use of Hydroxychloroquine, Azithromycin is effective and front-line physicians are using the therapy where permissible (13, 14, 15); and
Whereas, The COVID-19 pandemic is a serious medical issue, people are dying, and physicians must be able to perform as sagacious prescribers; therefore be it RESOLVED, That our American Medical Association rescind its statement calling for physicians to stop prescribing hydroxychloroquine and chloroquine until sufficient evidence becomes available to conclusively illustrate that the harm associated with use outweighs benefit early in
the disease course. Implying that such treatment is inappropriate contradicts AMA Policy H-120.988, “Patient Access to Treatments Prescribed by Their Physicians,” that addresses off label prescriptions as appropriate in the judgement of the prescribing physician (Directive to Take Action); and be it further RESOLVED, That our AMA rescind its joint statement with the American Pharmacists Association and American Society of Health System Pharmacists, and update it with a joint statement notifying patients that further studies are ongoing to clarify any potential benefit of hydroxychloroquine and combination therapies for the treatment of COVID-19 (Directive to Take Action); and be it further
RESOLVED, That our AMA reassure the patients whose physicians are prescribing hydroxychloroquine and combination therapies for their early-stage COVID-19 diagnosis by issuing an updated statement clarifying our support for a physician’s ability to prescribe an FDA21 approved medication for off label use, if it is in her/his best clinical judgement, with specific reference to the use of hydroxychloroquine and combination therapies for the treatment of the earliest stage of COVID-19 (Directive to Take Action); and be it further RESOLVED, That our AMA take the actions necessary to require local pharmacies to fill valid prescriptions that are issued by physicians and consistent with AMA principles articulated in
AMA Policy H-120.988, “Patient Access to Treatments Prescribed by Their Physicians,” including working with the American Pharmacists Association and American Society of Health System Pharmacists. (Directive to Take Action)
Fiscal Note: Modest – between $1,000 – $5,000
Received: 10/23/20
https://www.ama-assn.org/system/files/2020-10/nov20-handbook-addendum.pdf?fbclid=IwAR1HoUMyYjvKzEWVc1eUgQL25KmixgTiEthO9jEegimSpkJ6HvLIwjP_2gg
Here is second link from the AMA regarding the recommendation to NOT adopt the hydroxychloroquine resolution 509. It can be found on page 15.
https://www.ama-assn.org/system/files/2020-11/nov20-ref-com-e-annotated.pdf?fbclid=IwAR1X8fDRmzz5XrEQvLOEt2nFnCL9X66-1Y7BGB6i4hrmYFb42eEZ2O2UFNk
RESOLUTION 509 – HYDROXYCHLOROQUINE AND
COMBINATION THERAPIES – OFF-LABEL USE
RECOMMENDATION A:
Resolution 509 not be adopted.
RECOMMENDATION B:
Policy H-120.988 be reaffirmed.
HOD ACTION: Policy H-120.988 reaffirmed.
RESOLVED, that our American Medical Association rescind its statement calling for physicians to stop prescribing hydroxychloroquine and chloroquine until sufficient evidence becomes available to conclusively illustrate that the harm associated with use outweighs benefit early in the disease course. Implying that such treatment is inappropriate contradicts
24 AMA Policy H-120.988 that addresses off label prescriptions as appropriate in the judgement of the prescribing physician; (New HOD Policy) and be it further RESOLVED, that our AMA rescind its joint statement with the American Pharmacists Association and American Society of Health System Pharmacists, and update it with a joint statement notifying patients that further studies are ongoing to clarify any potential benefit of hydroxychloroquine and combination therapies for the treatment of COVID-19; (New HOD Policy) and be it further RESOLVED, that our AMA reassure the patients whose physicians are prescribing hydroxychloroquine and combination therapies for their early-stage COVID-19 diagnosis by issuing an updated statement clarifying our support for a physician’s ability to prescribe an FDA-approved medication for off label use, if it is in her/his best clinical judgement, with specific reference to the use of hydroxychloroquine and combination therapies for the treatment of the earliest stage of COVID-19; (New HOD Policy) and be it further
RESOLVED, that our AMA take the actions necessary to require local pharmacies to fill valid prescriptions that are issued by physicians and consistent with AMA principles articulated in AMA Policy H-120.988, including working with the American Pharmacists Association and
American Society of Health System Pharmacists. (New HOD Policy)
Your Reference Committee reviewed passionate and mixed testimony from both the online testimony and in the live hearing on this resolution.
our AMA Board of Trustees (BOT) provided testimony in opposition of this Resolution and supportive of the AMA statement. The BOT noted that several commentors misconstrued the language in the statement and outlined that it very clearly says, “Novel off-label use of FDA-
approved medications is a matter for the physician’s or other prescriber’s professional judgment” and also emphasized the need for physicians to rely on their professional judgment and medical evidence for any potential COVID-19 treatment option. The statement further notes that any use of these medications should be coordinated with a treating physician with
full understanding of the potential risks and benefits. The statement was accurate at the time it was issued and took the best evidence available into account. The BOT, CSAPH, and the majority of those who testified noted that while hydroxychloroquine has demonstrated benefits for multiple chronic autoimmune and rheumatologic diseases, the benefit for treatment of
9 COVID-19, at the time of the statement, had not been established, and that the AMA should base statements and policy on evidence and science. (How noble and high minded!) Many commentors, including the BOT and CSAPH noted that since the release of the statement several well-designed studies have failed to find benefit in the use of hydroxychloroquine for treatment of COVID-19 in multiple settings. (Canard or based on poor studies. What is benefit of not recording who made the statements and why did noone do conflict checks?) Several who testified also noted that it would be an embarrassment to the AMA and call the credibility of the AMA into question to rescind a statement that was evidence-based and accurate.
Those supportive of Resolution 509 noted that the statement was offensive to physicians and could undermine the patient-physician relationship. Your Reference Committee understands, and agrees with the need for physician autonomy, but also agrees with the BOT testimony that the AMA statement does not infringe on physician autonomy and thus should not be
21 rescinded. Your Reference Committee feels that AMA Policy H-120.988, “Patient Access to Treatments Prescribed by Their Physicians,” very clearly articulates the AMA’s strong support for autonomous clinical decision-making authority of physicians. Therefore, your Reference Committee recommends that Resolution 509 not be adopted and Policy H-120.988 be
reaffirmed.
H-120.988, “Patient Access to Treatments Prescribed by Their Physicians”
1. Our AMA confirms its strong support for the autonomous clinical decision-making authority of a physician and that a physician may lawfully use an FDA approved drug product or medical device for an off-label indication when such use is based upon sound scientific evidence or sound medical opinion; and affirms the position that, when the prescription of a drug or use of a device represents safe and effective therapy, third party payers, including Medicare, should consider the intervention as clinically appropriate medical care, irrespective of labeling, should fulfill their obligation to their beneficiaries by covering such therapy, and be
required to cover appropriate ‘off-label’ uses of drugs on their formulary.
2. Our AMA strongly supports the important need for physicians to have access to accurate and unbiased information about off-label uses of drugs and devices, while ensuring that manufacturer-sponsored promotions remain under FDA regulation.
3. Our AMA supports the dissemination of generally available information about off label uses by manufacturers to physicians. Such information should be independently derived, peer reviewed, scientifically sound, and truthful and not misleading. The information should be provided in its entirety, not be edited, or altered by the manufacturer, and be clearly distinguished and not appended to manufacturer-sponsored materials. Such information may comprise journal articles, books, book chapters, or clinical practice guidelines. Books or book chapters should not focus on any particular drug. Dissemination of information by manufacturers to physicians about off-label uses should be accompanied by the approved product labeling and disclosures regarding the lack of FDA approval for
such uses, and disclosure of the source of any financial support or author financial conflicts.
4. Physicians have the responsibility to interpret and put into context information received from any source, including pharmaceutical manufacturers, before making clinical decisions (e.g., prescribing a drug for an off-label use).
5. Our AMA strongly supports the addition to FDA-approved labeling those uses of drugs for which safety and efficacy have been demonstrated.
6. Our AMA supports the continued authorization, implementation, and coordination of the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. S